SESSION 1
THERAPEUTIC PROCESSES - I
Chairman: A. Shitzer
THE FUTURE OF BIOTHERMAL ENGINEERINGa
John C. Chatob and Raphael C. Leec
b Department of Mechanical and Industrial Engineering, Bioengineering
Faculty, University of Illinois, Urbana, Illinois 61801, USA
c Burn/Electrical Trauma Program, Department of Surgery, The University of
Chicago, Chicago, Illinois 60637, USA
ABSTRACT
This is a report on a three-day workshop held at the Allerton House of the
University of Illinois. The first day consisted of invited tutorials on topics related
to biothermal engineering: biological structures, analysis of microvascular heat
transfer, temperature measurement, cryobiology and cryosurgery, burns, and
industrial and consumer applications.
The rest of the workshop consisted of discussions in small groups and in plenary
sessions dealing with relevant topics. Although the discussions endeavored to be
as comprehensive as possible, the specific topics were selected by the
participants based on their expertise and interests.
The main areas examined were:
- Instrumentation
- Priority applications
- Mathematical modeling
- Thermal injury
The reliable measurement of the temperature distribution inside the living tissue
is still the premier problem of instrumentation although the measurement of other
parameters, such as properties, blood perfusion or heat flux, is also of great
importance.
The most important applications are medical, industrial, consumer, agricultural,
space, and military. The degree of sophistication needed in the analysis of
specific problems varies a great deal from relatively simple heat conduction
models to complicated ones including blood perfusion, anisotropy, and the
influence of large blood vessels. For many applications new experimental data
are still needed.
There have been significant advances in the modeling of living tissue with
increasing understanding of its thermal behavior. The consensus was, however,
that the models will always have to be tissue or organ specific and some new
models are still to be developed.
a This work was supported by the National Science Foundation through Grant No. CTS 96-
18518, and by the Bioengineering Faculty and the Department of Mechanical and Industrial
Engineering of the University of Illinois at Urbana-Champaign.
BLOOD PERFUSION MEASUREMENTS IN THE CANINE PROSTATE DURING TRANSURETHAL HYPERTHERMIA
Lisa X. Xua, Liang Zhub and Kenneth R. Holmesc
a School of Mechanical Engineering, Purdue University, West Lafayette, IN 47907-1288, USA
b Department of Mechanical Engineering, University of Maryland at Baltimore, MD 21250, USA
c Department of Veterinary Biosciences, University of Illinois, Urbana, IL 61801, USA
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a serious disease that generally occurs in
elderly men. Most of these individuals are in the high surgical risk group. As an
alternative to surgery, one of the recently developed therapeutic modalities is the local
hyperthermia induced either by the microwave or the radio frequency (RF) heating.
Histologically, prostatic hyperplasia develops spontaneously in both the dog and human.
Since the natural history of this condition in the dog is remarkably similar to that in the
human, the dog has been widely used in experimental studies to examine the
effectiveness of the microwave or the RF hyperthermia for BPH.
Recent studies on the transurethral-applied local hyperthermia in the canine and
human prostate have revealed significant effects of natural thermoregulation on the
therapeutical results. It has been long recognized that a major factor that affects tissue
temperature elevation and heterogeneity during hyperthermia, is the augmentation of
blood flow concomitant with the heating. In the present study, using the thermal pulse
decay (TPD) technique, blood perfusion rates were measured in different regions within
the canine prostate during transurethral heating. Relationships of the blood perfusion,
power deposition, and tissue temperature were observed and analyzed.
METHODS
During the microwave hyperthermia, fourteen male mongrel dogs (wt. 21.9 ± 3.1
kg) over four years old were used for blood perfusion studies. Dogs were anesthetized
using Na-pentobarbital, i.v. (30 mg/kg). The bladder and prostate were exposed through a
midventral abdominal incision. A small cut was made in the bladder wall to allow
insertion of the transurethral thermal therapy (T3) catheter (Urologix, Inc. MN) into the
prostatic urethra. Within the catheter, a microwave antenna is located approximately in
the center and chilled water at a given temperature flows between the antenna and the
inner catheter wall. A fiber optic thermosensor built inside of the catheter monitored the
prostatic urethral wall temperature throughout each experiment.
Thermistor bead probes of different lengths were placed at various locations
within the prostatic tissue. These probes serve two purposes: thermal pulse delivery and
local temperature measurement. The blood perfusion rate and tissue thermal conductivity
was measured simultaneously in the canine prostate using the thermal pulse decay (TPD)
technique. This technique is based on a comparison of the measured with the model
simulated temperature decay following a heating pulse delivered by a thermistor bead
probe. A solution of the Pennes bio-heat transfer equation is used to construct the
theoretical model relating local blood perfusion to the temperature decay.
To study the instantaneous blood perfusion response to the local tissue
temperature increase, the RF heating was applied to the canine prostate using the EESY-
100 RF Prostatic Hyperthermia System (Yuanshui Industrial Co. PRC). The use of the
RF heating was intended in this part of the study to ensure a broad uniformly heated
tissue region for examination of the blood perfusion response. Nine male mongrel dogs
(wt. 21.9 ± 3.1 kg) over four years old were used. A thermocouple built inside the RF
catheter was used to monitor the urethral wall temperature throughout the entire
experiment.
Experimental data are shown as mean ± standard deviation (SD). Differences
among the mean values were determined by one-way repeated measures ANOVA using
SYSTAT software. The post hoc comparisons between any two levels were performed by
the modified student t-test.
RESULTS AND DISCUSSIONS
Microwave Heating
Results indicate that, under the normal condition, the periurethral region is most
highly perfused with an average rate of 0.60±0.25ml/min/gm (n=4) while the perfusion
rate is 0.34±0.22ml/min/gm (n=10) in parenchyma. An approximately 3.5 fold increase in
perfusion from the respective baselines was observed in both regions when the local
tissue temperature was raised to 41.5oC by the microwave heating. Another 0.5 fold
increase was found in parenchyma after the tissue was further heated to 43.1oC at which
oscillatory behaviors in tissue temperature have been observed. In general, the present
baseline perfusion falls within the range of 0.20 - 0.79 ml/min/gm, the measured average
perfusion rate throughout the entire canine prostate via different techniques from
previous studies. Use of the TPD technique in this study has enabled us to examine blood
perfusion in different regions within the prostate. The periurethral perfusion was
significantly higher than that in the parenchymal region at the baseline and 10W
microwave heating level. This could be partially attributed to the fact that in the prostate
gland, the periurethral region is supplied by the artery of the urethral bulb while the
parenchyma is perfused by radial tributaries from the subcapsular artery passing along the
capsule septa toward the urethra. Thus, the baseline and the response of blood perfusion
to the microwave heating would likely be different in these two regions.
RF Heating
As revealed earlier, there was no significant increase in perfusion observed in the
prostate under the 5W microwave heating. Blood perfusion response to the RF heating
was therefore observed only at the 10W and 15W level. Two interesting phenomena have
been found. First, the oscillatory temperature behavior seems to be coupled with an
oscillatory change in blood perfusion. Within each cycle, the change in perfusion appears
to be closely related to not only the tissue temperature but also the temporal temperature
gradient. Second, the maximal perfusion and interstitial temperature occurred almost at
the same moment that was several minutes behind the time when the maximal urethral
wall temperature was reached. Since both the urethral wall and interstitial temperatures
are regulated by blood perfusion, it is not difficult to see that blood perfusion acts as a
feedback of the local tissue temperature in a closed control system.
CONCLUSION
In this study, a close relationship of blood perfusion to local tissue temperature
and its temporal gradient has been observed. It seems that all the physiological factors
which influence the change of blood flow, are most likely stimulated by the thermal field
rather than the non-thermal effects from the electromagnetic fields. Results from this
study will not only help to improve the efficacy of hyperthermia treatment, but more
importantly to provide a better understanding of thermoregulation in biological systems
during hyperthermia.
ACKNOWLEDGMENT
This research was supported by NIH 5 R29 CA67970. Authors wish to thank
Urologix, Inc. and Yuanshui Industrial Company for providing the transurethral thermal
therapy systems and technical assistance to this research. Many thanks are also extended
to Mr. David Y. Yuan and Dr. Lie-Wen Pang for their great assistance to this
experimental study.
MATHEMATICAL SIMULATION OF HEAT TRANSFER PROCESS IN SKIN COVER AT BURN INJURY
R.Sh.Enalejev, W.A.Kachalkin
Department of Cybernetics, Kazan Chemical Technological University, Russia, K. Marx Street,68,
Kazan 420015
INTRODUCTION
In everyday life, during high intensity heating technological processes, receiving and storing of highly
inflammable fuels and explosives one can always run into the danger of getting burn injury under
intensive heat influence.
Purposeful search of heat protection materials is impossible without testing them with reliable
quantitative estimation of human injuring level.
The most objective estimation may be received during natural tests, but such tests demand
considerable material and intellectual expenditures.
The most widely used laboratory methods of testing of heatprove characteristics of cloth is the
standard method TPP (Thermal Protective Performance Test). According to this method critical
energy is used as burn injury criterion [1].
Analysis of literature data [1-5] shows, that threshold meaning of heat impulse, causing injury is a
variable quantity. Range of changing of this quantity at intensive heating influence may be
considerable.
It is known the appearance of injury and changes connected with it in organism depend on intensity
and depth of tissue heating [3].
However results of temperature profile measuring in skin of warm-blooded animals are not given in
literature. Application of tissue thermometric method for this purpose is not correct, because
dimensions even of a small temperature sensoring element are compared with thickness of heated
skin level.
Therefore, for determination of temperature profile in skin cover and basing of temperature criterion
of thermal burns it is necessary to search other methods. One of them may be the method of
mathematical modeling.
In this study, a complex method is suggested for determination of skin temperature field; it includes
mathematical simulation of heat transfer process in skin cover during the time of influence of thermic
agent and experimental reproduction of burn injury with measurement of given temperature on
animal body surface done beforehand.
MATHEMATICAL FORMULATION
For calculation of temperature according to depth of skin cover we carried out mathematical model
of heat transfer process. In this model the difference of thermophysical properties of structural skin
layers was taken into account.
Mathematical description of model may be represented in the following way. Three-layered cylinder,
with different coefficients of layer temperature conductivity has the initial temperature T = 37o C.
During period of time  - time of thermo agent influence, causing second-degree
burn) temperature of external surface (x = 0) under influence of heat source is mounted equal to T1.
In region - time of thermo agent influence, causing second-degree
burn) temperature of external surface (x = 0) under influence of heat source is mounted equal to T1.
In region  it is necessary to build the solution of differential heat
conductivity equation it is necessary to build the solution of differential heat
conductivity equation
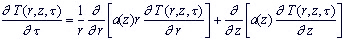 ...(1) ...(1)
satisfying the following initial and boundary conditions
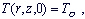 ...(2) ...(2)

at 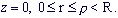 ...(3) ...(3)
at the rest part of bounder of area (except line r=0)
 ...(4) ...(4)
where n - normal to area bounder.
CALCULATION RESULTS AND DISCUSSION

The program of initial task solution is composed so that it allows to receive temperature distribution
in skin cover at any low of temperature change on body surface.
The mathematical modeling of heat distribution in skin cover layers for different geometric sizes of
heated zones r, R & z and different time of increased temperature t* influence was carried out. The
results of modeling have shown that the sizes of the central part of the spot on which condition of
one dimensional heat distribution are 0.7 - 0.9 r during all period of heating.
That is why in the experiments with burns, the size of round heating element were 20 mm. Inside of
this spot in zone with diameter 15 mm the temperature difference between one dimensional and two
dimensional cases is not more than 3 %. That is considerably less than statistic mistake of burn
reproduction. On the other hand, the spot with diameter 15 mm is quite sufficient for clinic
estimation of burn injury.
Changes of temperature in each structural skin layer to the time moment t* are shown as example in
Fig. 2. These changes were received by calculation process for experimental meanings of surface
temperatures.
From presented results it is seen that irrespective of temperature profile in studied range of surface
temperatures second-degree burn and more appear in time period, when temperature of nipple layer
of dermis reaches the critical magnitude (49oC).
According to data of physiologists, that is the exact temperature when the death of heat receptors in
derma occurs, and it seems to be the reason of breach of physiologic skin thermoregulation, that
leads to pathologic changes.
SUMMARY
- Critical temperature of skin cover under epidermis boundary causing second-degree injury was determined by method of mathematical simulation.
- The instrumental method of heatprotecting material properties evaluation was suggested with the usage of critical temperature criterion.
REFERENCES
- Behnke W.P., Predicting Flash Fire Protection of Clothing from Laboratory Tests Using Second-degree Burn to Rate Performance. Fire and Materials., Vol.8, No. 2, pp 57-63, 1984.
- Stoll A.M., Hardy J.D., Greene L.C., J. Appl. Physiol., 15, 489, 1960.
- Stoll A.M. "The Role of Skin in Heat Transfer" in "Advances in Heat Transfer", (ed. by Hartnett, J.P. and T.F. Irvine), Academic Press, New York, , Vol. 4, p.115, 1967.
- Stoll A.M., and Chianta M.A., Method and Raiting System for Evalution of Thermal Protection, Aerosp. Med. 40 (11), pp 1232-1238, 1969.
- Barker R.L. and Lee Y.M. Analyzing the Transient Thermophysical Properties of Heat-resistant Fabrics in TPP Exposure, Textile Research Journal, June, pp 331-338, 1987.
|

