SESSION 5
MASS TRANSFER - I
Chairman: J. Chato
FLUID FILTRATION ACROSS THE ARTERIAL WALL UNDER FLOW CONDITIONS: IS WALL SHEAR RATE ANOTHER FACTOR AFFECTING FILTRATION RATE?
Xiaoyan Dend, Yves Marois, Robert Guidoin
Department of Surgery, Laval University, Ste-Foy, Québec, Canada G1K 7P4 and
Québec Biomaterials Institute, Inc., CHUQ, Québec, Québec, Canada G1L 3L5
INTRODUCTION
Pressure-driven fluid convection or filtration flow across the artery wall may promote
lipid infiltration,1 therefore having important implications in the earliest stage of
atherogenesis.2
Filtration flow across large arteries has been studied by many investigators, and it is
now common knowledge that endothelial lining integrity3,4 and transmural pressure5,6 are
two major factors affecting filtration flow. However, the filtration rate across the wall of
an artery has also been found to depend on the perfusate albumin concentration, by
decreasing according to increasing albumin concentration in the perfusion solution.7 This
dependency has been attributed mainly to a phenomenon called 'concentration polarization'
at the blood/wall interface with the macromolecules increasing in concentration from the
bulk value towards the interface.7
Concentration polarization of macromolecules is influenced by the flow rate, or wall
shear rate, and drops when wall shear rate increases.8,9 Therefore we have reason to
believe that the flow rate or wall shear rate may also affect the filtration flow across the
artery wall. The previous measurements of filtration flow or hydraulic conductivity of
blood vessel walls were made in the absence of blood flow, which is not the case in vivo.
In the present study, the filtration rates across the canine carotid artery were measured in
vitro under 4 different flow conditions.
MATERIALS AND METHODS
The canine common carotid artery was cannulated with thin stainless steel pipes and
then encased in a water tight transparent Plexiglas chamber (Fig. 1). The encased blood
vessel segment was perfused with Krebs solution in steady flow. Filtration rates across the
walls of the freshly prepared arterial segments were measured using the calibrated glass
capillary on the chamber under 4 different experimental conditions: 1) albumin-free Krebs
solution under absent flow condition; 2) albumin-free Krebs solution with flow (148 ± 8
ml/min); 3) Krebs solution containing 1.0 g/dl bovine serum albumin under absent flow
condition; 4) Krebs solution containing 1.0 g/dl bovine serum albumin with flow (148 ± 8
ml/min).
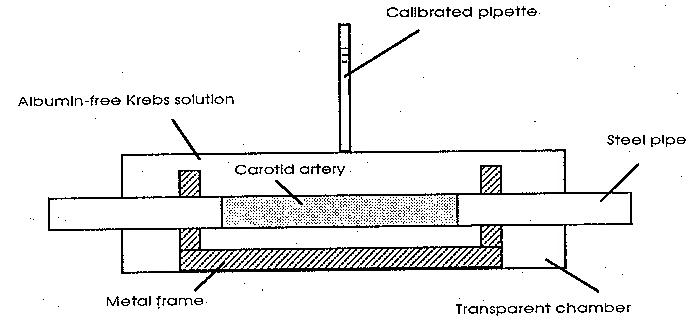
Figure 1. Schematic drawing of the specimen chamber used to measure the filtration rates. The
pipette was calibrated by injecting known aliquots of solution.
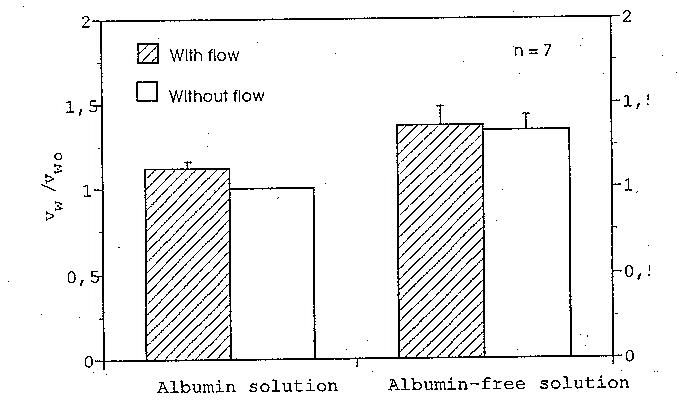
Figure 2. Mean values of the ratios between the filtration rates under different experimental
conditions. vwo is the filtration rate of the albumin solution under absent flow conditions. Note
that the fluid flow only affects the filtration rate of the albumin solution; its influence on the
filtration rate of the albumin-free solution is not significant.
RESULTS
The mean values of the normalized filtration rates with standard deviations are plotted
in Fig. 2. The filtration rate of the albumin solution under a flow rate of 148 ml/min was
about 12% higher than that registered under absent flow conditions (p < 0.005). In the case
of the albumin-free solution, the statistics showed no significant difference in filtration rate
between the flow condition and the absent flow condition (p > 0.01), indicating that the flow
did not affect the filtration rate of albumin-free solution across the artery wall.
DISCUSSION
Due to the fact that flow has no effect on the filtration rate across the arterial wall in
the absence of albumin, we doubt that the changes in filtration rate were caused by a
cellular response to wall shear forces. We do, however, suggest that the increase of
filtration rate was basically the result of the decreased luminal surface concentration of
albumin. Albumin protein particles on the luminal surface affects the filtration flow
twofold: 1. The concentrated protein layer creates a greater osmotic pressure difference
across the wall of the tested vessel, thereby lowering the pressure gradient across the artery
wall, which is the driving force for filtration flow. 2. Most importantly, albumin particles
accumulated on the luminal surface of the test artery can physically block the junctions
and pores of the endothelium, hindering the passage of fluid through the wall. The luminal
surface concentration of macromolecules is influenced by the flow rate, or wall shear rate,
and drops when wall shear rate increases. That is why filtration rate of the albumin
solution (but not the albumin-free solution) across the arterial wall increased under flow
conditions.
In conclusion, concentration polarization (luminal surface concentration) of proteins
or lipoproteins has a significant effect on filtration across the artery wall. Luminal surface
concentration of protein particles is wall shear-dependent. Therefore, blood flow or wall
shear rate may indeed affect the filtration rate of the artery wall.
REFERENCES
- Tedgi, A. & M.J. Lerver. 1985. The interaction of convection and diffusion in the transport
of 131I-albumin within the media of the rabbit thoracic aorta. Circ. Res. 57: 865-873.
- Walton, K.W. 1975. Pathogenetic mechanism in atherosclerosis. Am. J. Cardiol. 35: 542-
558.
- Vargas, C.B., F.F. Vargas, J.G. Pribyl & P.L.Blackshear. 1979. Hydraulic conductivity of
the endothelial and outer layers of the rabbit aorta. Am. J. Physiol. 236: H53-H60.
- Tedgui, A. & M.J. Lever. 1984. Filtration through damaged and undamaged rabbit thoracic
aorta. Am. J. Physiol. 247: H784-H791.
- Lever, M.J. & N. Sharifi. 1987. The effects of transmural pressure and perfusate albumin
concentration on the hydraulic conductivity of isolated rabbit common carotid artery
(abstract). J. Physiol. 387: p. 68.
- Baldwin, A.L., L.M. Wilson & B.R. Simon. 1992. Effect of pressure on aortic hydraulic
conductance. Ateriosclerosis and Thrombosis 12: 163-171.
- Tarbell, J.M., M.J. Lever & C.G. Caro. 1988. The effect of varying albumin concentration
on the hydraulic conductivity of the rabbit common carotid artery. Microvasc. Res. 35:
204-220.
- Blackshear, P.L., K.W. Bartel & R.J. Forstrom. 1977. Fluid dynamic factors affecting
particle capture and retention. In The Behavior of Blood and Its Components at
Interface. L. Vroman & E.F. Leonard, Eds: 270-279. New York Academy of Science.
New York.
- Deng, X.Y., Y. Marois, T. How, Y. Merhi, M.W. King & R. Guidoin. 1995. Luminal
surface concentration of lipoprotein (LDL) and its effect on the wall uptake of
cholesterol by canine carotid arteries. J. Vasc. Surg. 21: 135-145.
MATHEMETICAL MODEL FOR CONTROLLED DIFFUSIONAL RELEASE
OF DISPERSED SOLUTE DRUGS FROM
MONOLITHIC IMPLANTS
R. Collins*, N. Jinuntuya, P. Petpirom and S. Wasuwanich
Wright State University*, Department of Biomedical Engineering, Dayton, Ohio 45435 (U.S.A.)
and Kasetsart University, Departments of Mathematics & Physics, Bangkok 10900 ( Thailand)
ABSTRACT
New mathematical models are formulated and analytical solutions are presented for the
diffusional release of a solute from a biodegradable drug-impregnated slab matrix in which the
initial drug loading c0 is greater than the solubility limit cs. A Stefan problem with moving
boundaries results from this formulation. An inward moving diffusional front separates the
reservoir (unextracted region) containing the undissolved drug from the partially extracted region.
The cumulative mass released is determined as a function of time. The ultimate goal of such an
investigation is to provide a reliable design tool for the fabrication of specialized implantable
matrix/drug combinations to deliver pre-specified and reproducible dosages over a wide spectrum
of conditions and required durations of therapeutic treatment. Such a mathematical/computational
tool may also prove effective in the prediction of suitable dosages for other drugs of differing
chemical or molecular properties without additional elaborate animal testing.
Controlled-release drug delivery is a subject of keen interest world-wide at this time. National
and international pharmaceutical concerns and biomedical device developers are actively interested
in exploiting this rapidly evolving and potentially lucrative market. Some of the many state-of-the-
art applications may conceivable include:
- sustained release of estrogen and progesterone for menopausal women
- programmed release of antibiotics for patients recovering from surgical repair of bone
fractures and/or installation of articular prostheses
- encapsulation of ovarian cells which themselves may continue to produce estrogen
- slow release of contraceptive chemicals for both men and women, particularly in third-world
countries where the discipline of taking daily doses may be lacking
- drug delivery to targeted organs: e.g. slow release of insulin for diabetics
- encapsulation of pancreatic cells which may themselves trigger the natural production of
insulin on a long term self-sustainable basis for diabetic patients.
Recent technical advances now permit one to control the rate of drug delivery. The required
therapeutic levels may thus be maintained over long periods of months and years through implanted
rate-controlled drug release devices. Two such novel drug delivery systems currently employed are
implantable polymeric and ceramic erodible monoliths.
An inward moving diffusional front separates the reservoir (unextracted region) containing the
undissolved drug from the partially extracted region. This front is followed by an inward
propagating erosion front. The positions of these fronts are not known a priori but must be
determined in the course of the solution. The mathematical formulation of such moving boundary
problems (Stefan problem) has wide application to heat transfer with melting phase transitions and
diffusion-controlled growth of particles, in addition to our topic of controlled-release drug delivery.
Additional applications of an industrial, agricultural or environmental nature, involving the
diffusional release of a dispersed or dissolved solute from a polymeric monolith in which a pre-
programmed dose-time schedule is necessary, further extend the interest in this problem to
international levels. Examples of such applications may include the removal of solvent from
polymer solutions during the dry spinning of fibres, photoresist technology and microlithography,
diffusional release of pollutants and additives from polymers into the environment and controlled
release of agricultural chemicals.
The rate-limiting property of the controlled drug delivery system resides in the design
properties of the implanted drug delivery device itself, and is not necessarily dependent upon the
physiology of the subject. Drug release kinetics may depend on a number of intrinsic properties of
the drug delivery implant: drug solubility, molecular weight and partition coefficient, matrix
swelling, osmotic pressures, ion exchange, local electromagnetic force fields, etc. In this work, we
focus on two specific properties of the drug delivery system: drug diffusion and matrix erosion. By
the term erosion, we mean to denote the process by which material which is intrinsically insoluble
in water can be converted into one that is water-soluble. In a pendant chain system for example,
one may imagine a drug which is covalently bound to insoluble molecules and is then released by
scission of its bonds either by water or by enzymatic action. As the surface of the matrix is
exposed to the surrounding extracellular fluids, its structure erodes and the drug impregnated
within its walls and pores escapes.
We obtain simplified expressions based upon linearized distributions of drug concentration.
These are valid under conditions of pseudo-stationarity; that is, when the velocity of the diffusion
front is not too large as to alter concentration distributions too abruptly. Outside the pseudo-
stationary range, the diffusion front progresses more rapidly through the matrix medium and the
pseudo-stationary approximation upon which the linearized concentration distributions were based
may no longer be valid. We have shown that the analytical solutions using linear concentration
distributions are accurate to within a few percent of our exact numerically obtained solutions for a
wide range of the dimensionless parameters a and b , where a is the ratio between the solubility
limit concentration of the drug behind the diffusion front and the initial loading concentration of the
drug in the matrix ahead of the diffusion front, and the dimensionless parameter b is the ratio of
the erosion front velocity times the initial thickness of the slab divided by diffusion coefficient. The
exact numerical results were obtained by a straightforward finite-difference computational solution
of the governing equations with given initial and boundary conditions.
Thirty-one cases were computed with the following parameter values: Set 1: three values of
b = 0.05; .08; 0.10 with five values of a = 0.08; .10; 0.12; 0.18 and 0.20 (15 possible
combinations) and Set 2: four values of b = 0.1; 0.5; 1.0 and 5.0 combined with four values of
a = 0.5; 1.0; 2.0 and 10.0. In this range of practical interest, the agreement of the linear
approximation with the exact numerical solution for the position and velocity of the diffusion front
and the resulting cumulative mass release as a function of time is seen to be very adequate. The
analytically derived cumulative mass curves begin gradually to diverge from the exact
comptational solutions for a > 0.5.
By adjusting several design parameters characterizing the drug delivery system, one may
control the cumulative mass release process in a predictable manner. For example, by
incorporating the desired macromolecular drug into the casting of a polymeric matrix, "winding
tortuous" pores are created which condition the speed of the diffusion process through a
corresponding alteration in the effective permeability of the cast drug-impregnated matrix. By
coating the matrix with an impermeable film or membrane, one can slow down the rate of drug
release until bioerosion occurs. One may also increase the diffusion rate by raising the ratio of
drug loading to solubility limit co/cs.
Many of these considerations, including the shape factor and matrix swelling, can be
incorporated into further refined versions of the above models which may then serve as a valuable
framework for the design of experimental protocols to test and evaluate the sensitivity of such
factors on the cumulative mass release of drug into the body as a function of time. Currently, time-
consuming trial-and-error methods are employed in the design of such implants. Using the present
approach, an efficient computationally aided tool can be created to design the desired implant in
order to release the clinically prescribed dose-time program for specific therapeutic applications
ranging from weeks to years in duration.
|

